Recently, Professor Lingna Sun’s team from the College of Chemistry and Environmental Engineering of Shenzhen University published the latest research progresses about Sodium-Ion batteries in Small.
Among the numerous potential anode materials for sodium-ion batteries (SIBs), alloying metal-based chalcogenides with conversion-alloying-type reactions are attracted attentions, such as tin, antimony and zinc chalcogenides, which exhibit higher specific capabilities associated with conversion-alloying combined mechanism and improved electrical conductivity better than metal oxides. However, every coin has two sides, the alloying-type and conversion-type behaviors do bring high reversible capacities, at the same time make the anode structures easier to degrade unavoidably, which are generally accompanied with volume expansive deformation and active material pulverization leading to electric contact degradation peeling from the current collector.
In this article, ZnSn(OH)6 nanobox precursors were first electrospunned with polyacrylonitrile (PAN) to obtain a 3D free-standing network, after thermal treatments with carbonization and selenylation, ZnSe/SnSe heterostructures embedded in the Se/N co-doped CNF skeleton have been developed (ZnSe/SnSe@Se,N-CNFs). The unique ZnSe/SnSe heterostructures, electrospun CNF and Se/N co-doped characteristics can effectively promote electron/Na+ diffusion rate, enhance the structure stability, and accelerate the electrochemical reaction kinetics: I) The hetero-boundaries in the ZnSe/SnSe will provide enhanced interfacial coupling effect and built-in electric field with additional charge-transfer driving force, which can attract more Na+ ions to adsorb in the heterogeneous interfaces and improve the migration rate of Na+ ions from electrolyte to electrode, thereby effectively accelerating the electrochemical kinetics. II) The 3D free-standing CNF network embedded with ZnSe/SnSe nanoboxes, can not only enhance the conductivity of semiconductor ZnSe/SnSe but also improve its structure integrity avoiding violent volume expansion/shrinkage during the insertion/extraction process. III) The Se/N co-doping feature achieves a coupling effect between the CNF matrix and the selenide heterostructures, enabling fast ion/electron transport and accelerating surface/internal reaction kinetics for Na-ion storage, and enhancing the electronic conductivity and wettability of the CNF matrix. This research gives some inspiring design ideas for addressing the current challenges of alloy-based materials when applying in sodium-ion batteries: The electrical conductivity has been enhanced by hetero-nanostructure construction, carbon-shell coating, and co-doping modification strategies; and volume expansion issue has been addressed by embedding alloyed materials into carbon matrix with 3D free-standing skeleton feature.
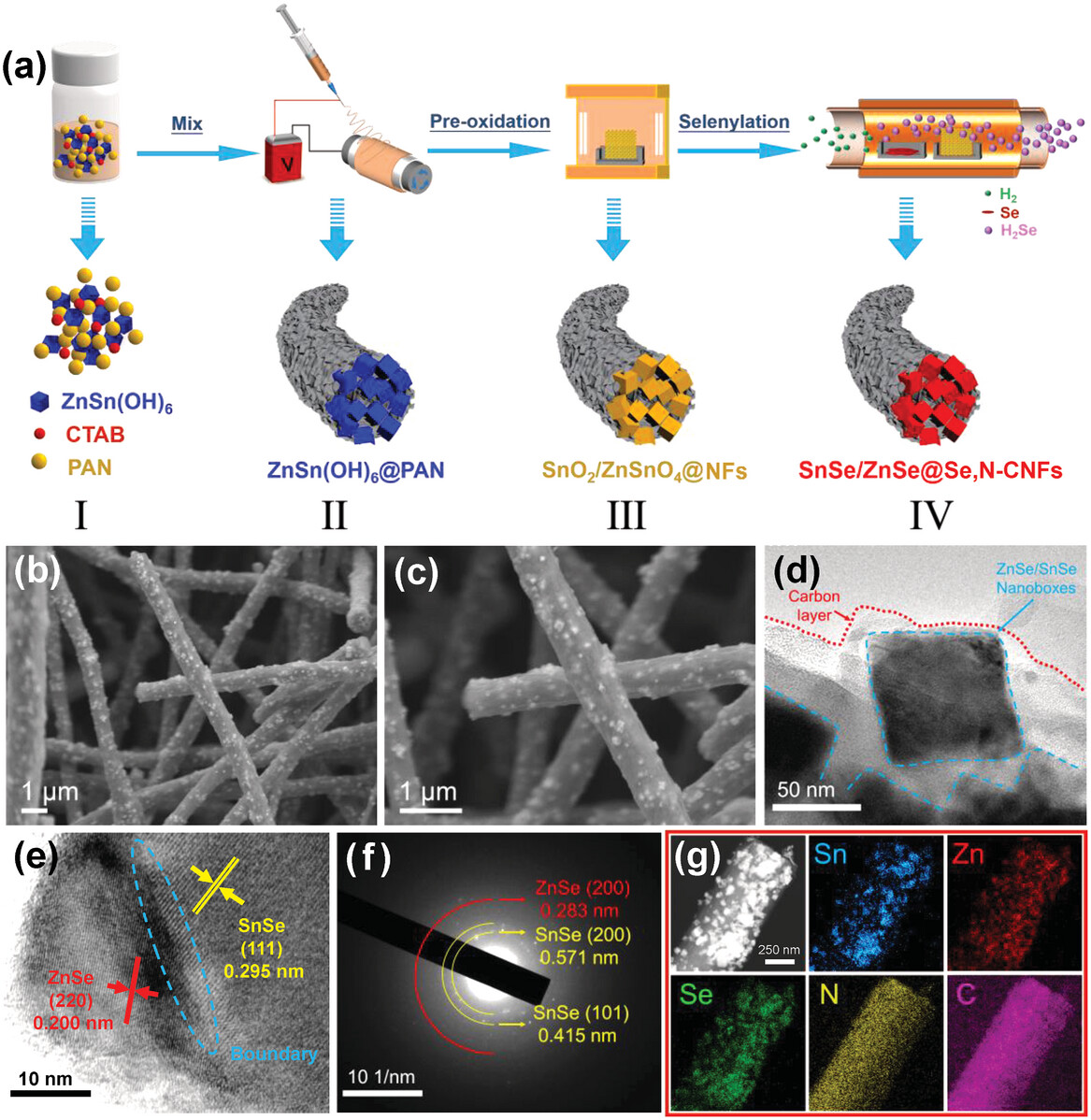
图
1
ZnSe/SnSe@Se,N-CNFs
合成过程示意图和形貌表征
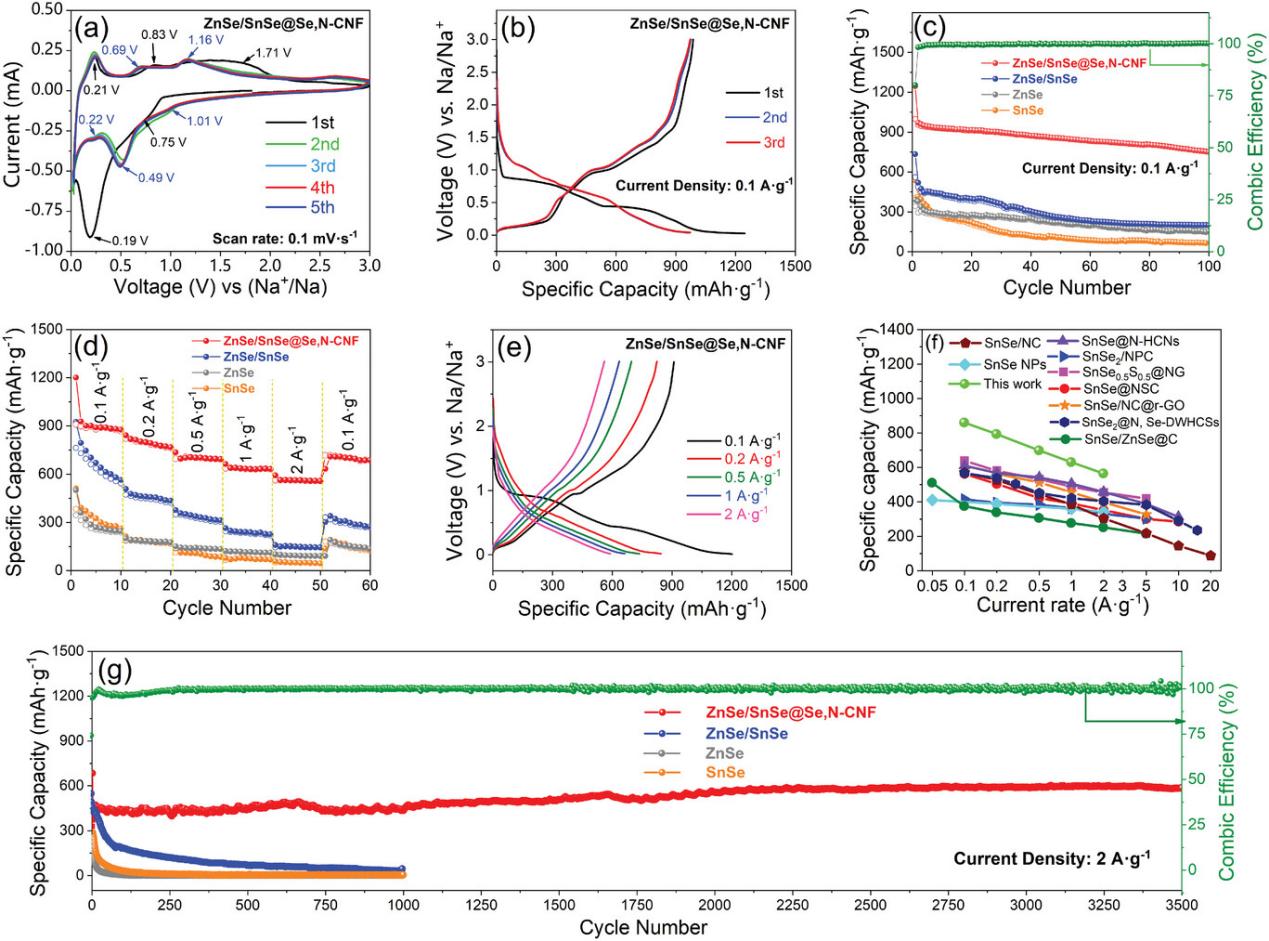
图
2
ZnSe/SnSe@Se,N-CNFs电极
的电化学性能
The research article “ZnSe/SnSe Heterostructure Incorporated with Selenium/Nitrogen Co-Doped Carbon Nanofiber Skeleton for Sodium-Ion Batteries” was published in Small (IF: 13.3, JCR Chemistry Region 2, TOP Journal). Researcher Yingmeng Zhang and Postgraduate student Lele Cheng are the first authors of this paper; Researcher Yingmeng Zhang and Professor Lingna Sun are the corresponding author. College of Chemistry and Environmental Engineering of Shenzhen University is the corresponding unit.
This work was financially supported by National Natural Science Foundation of China , Shenzhen Science and Technology Foundationand Guangdong Basic and Applied Basic Research Foundation, China.
See full text link: https://doi.org/10.1002/smll.202306536
