Hydrogen energy is a zero-carbon and environment-friendly energy with a high energy density. Renewable electricity-drived electrocatalytic water splitting is an environmentally friendly and efficient strategy to large-scale produce hydrogen. Electrocatalytic water splitting mainly includes cathodic HER and anodic oxygen evolution reaction (OER). However, the inherent disadvantages of OER, including a high theoretical potential (1.23 V) and sluggish kinetics, renders the two-electrode water electrolyzer often require a cell voltage of 1.6–2.0 V much higher than the theoretical potential value (1.23 V) to achieve a preferable current density, greatly increasing the energy consumption of water splitting. To reduce the energy consumption of hydrogen production, it is highly desirable to replace the anodic OER by the oxidation reaction of small organic molecule with relatively low theoretical potentials. It is found that the HzOR with a low theoretical potential (–0.33 V) is a promising alternative to the OER for achieving energy-saving hydrogen production. Another benefit is that the product of HzOR is only nitrogen without carbon emission, and the mixing of anodic N2 and cathodic H2 products will not cause safety problems.
Lattice strain engineering has emerged as an effective and versatile strategy to enhance electrocatalytic activity of various electrocatalysts including precious and non-precious metal-based electrocatalysts due to its powerful capacity for tuning binding energies of reaction intermediate. The continuously modulating of lattice strains of non-precious metal-based electrocatalysts may also flexibly tune the adsorption strength with reaction intermediates of the HzOR and HER for reaching the vertex of adsorption “volcano plot”. However, the related studies are still few and limited in scope.

Recently, distinguished professor Chuanxin He of the College of Chemistry and Environmental Engineering, Shenzhen University published the latest research progress on 《 Advanced Materials 》 (IF:29.4, JCR Chemistry Region 1, TOP Journal). The title is 《Lattice Strain Engineering of Ni2P Enables Efficient Catalytic Hydrazine Oxidation-assisted Hydrogen Production》. The first author of this paper is Chao Feng, a postdoctoral researcher, and Miaoyuan Lyu, a postgraduate student, and the corresponding author is Chuanxin He distinguished professor and Qi Hu associate professor. College of Chemistry and Environmental Engineering of Shenzhen University is the corresponding unit.
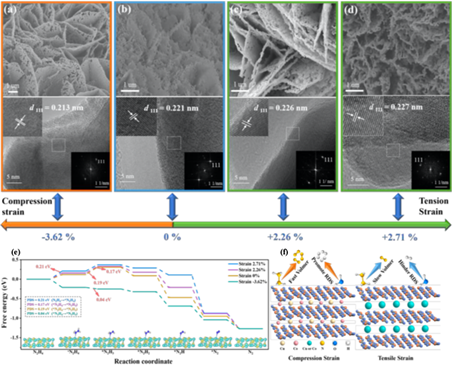
Figure 1. SEM (top) and HRTEM (bottom) images of (a) Cu1Co2–Ni2P/NF (−3.62%), (b) Ni2P/NF (0%), (c) Co–Ni2P/NF (+2.26%), and (d) Cu–Ni2P/NF (+2.71%). DFT calculations. (e) The free energy profiles of HzOR on Ni2P/NF, Cu1Co2–Ni2P/NF, Co–Ni2P/NF, and Cu–Ni2P/NF, with 0%, −3.62%, +2.26%, and +2.71% strains, respectively, and the Ni2P strain model for adsorption of corresponding intermediates. (f) Schematic display the enhanced effect of suitable compressive strains for Ni2P-catalyzed both the HzOR and HER.
This work developed a facile yet robust strategy for lattice strains engineering of Ni2P via the dual cation-doping, thereby achieving significantly enhanced electrocatalyticperformance for both the HzOR and HER. As a result, the optimized electrocatalyst of Cu1Co2–Ni2P with a lattice compressive strain of 3.62% showed operational potentials of −0.052 and −0.051 V vs. RHE at the current densities of 10 and –10 mA cm−2 for the HzOR and HER, respectively. Notably, as a bifunctional electrocatalyst for hydrazine-assisted water splitting, Cu1Co2–Ni2P required operating voltages of merely 0.16 and 0.39 V to reach current densities of 10 and 100 mA cm−2, respectively. DFT results uncovered that the lattice compression strain induced by the double cation-doping could effectively optimize the electron configuration of Ni2P, regulate the d-band center, and considerably reduce the reaction energy barrier of HzOR and HER. These results demonstrate that the simple dual-cation doping provided here might be extended to develop a large family of advanced electrocatalysts with tunable lattice strains towards various important electrocatalytic reactions, i.e., carbon dioxide reduction, and oxygen reduction reactions.
See full text link:https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.202305598
